1027
Views & Citations27
Likes & Shares
Alternative strategies are urgently required to fight obesity and associated metabolic disorders including diabetes and cardiovascular diseases. Brown and brown-like beige adipocytes (BAs) store fat, but in contrast to white adipocytes, they are equipped to dissipate energy stored. Therefore, BAs represent promising cell targets to counteract obesity. However, the scarcity of BAs in adults is a major limitation for a BA-based therapy of obesity, and the notion to increase the BA mass by transplanting BA progenitors (BAPs) in obese patients recently emerged. The capacity of human induced pluripotent stem cells (hiPSCs) to generate BAPs at a high efficiency offers the opportunity to produce an unlimited number of patient-matched BAs. However, hiPSC-BAPs display a low adipogenic capacity that hampered their use both in cell-based therapy and basic research. Recently we, and others, have identified the critical role of TGFβ pathway in switching off differentiation of hiPSC-BAPs in classical 2D culture and also in a 3D beige adiposphere model better mimicking adipocytes in vivo. Inhibition of TGFβ pathway unlocks differentiation of hiPSC-BAPs making this cell model a suitable tool for therapeutic transplantation. In contrast to BAPs derived from human iPSCs, inhibition of TGF β pathway is not a requisite for differentiation of preadipocytes derived from adult adipose tissues. This observation suggests that hiPSC-BAPs and adult adipose tissue-preadipocytes are at different stages of the adipose progenitor hierarchy.
Keywords: TGFβ pathway, Human induced pluripotent stem cells, Beige adipocytes, Adipocyte progenitors, Stem cell-based therapy, Obesity
Abbreviations: TGF: Transforming Growth Factor; BA: Brown and Beige Adipocyte; BAP: Brown and Beige Adipocyte Progenitor; hiPSC: Human Induced Pluripotent Stem Cell
THE TGF β PATHWAY GOVERNS THE DIFFERENTIATION OF hiPSCs INTO BAs
Induced pluripotent stem cells (iPSCs) represent an abundant source of multiple cell types of therapeutic interest for drug screening as well as for transplantation [8,9]. Nakao’s group was the first to demonstrate the capacity of hiPSCs to generate white adipocytes [10]. Total differentiated hiPSC populations, but not purified adipose progenitors, were transplanted into mice. Indeed, differentiated hiPSC cultures can be enriched with adipocytes, but still contain other cell types that are unsuitable for transplantation, including undifferentiated hiPSCs that can form teratomas. An alternative to eliminate hiPSC capacity to form teratomas consists in purifying progenitors of interest during hiPSC differentiation. Ahfeldt et al. [11] and Mohsen-Kanson et al. [12] were able to generate pure BAs from hiPSCs that displayed a high adipogenic capacity but only following transduction with adipogenesis master genes. The need to genetically modify hiPSCs-derived progenitors to generate adipocytes clearly illustrates the low adipogenic potential of hiPSCs. This feature represented a bottleneck hampering their clinical use [13].
Several factors, such as ascorbic acid, EGF and hydrocortisone have been shown to regulate hiPSC-BAPs differentiation [12,14,15]. However, TGFβ signaling holds a pivotal role. Members of the TGFβ family are expressed in various tissues where they have been shown to regulate various biological processes including regulation of apoptosis, proliferation and differentiation of different cell types [16]. The TGFβ pathway emerged as a critical anti-adipogenic player through the activation of Smad 2/3 [17-19]. Deletion of TGFβ receptor 1 in mice has been shown to promote beige adipogenesis within white adipose tissue, supporting a model where TGFβ receptor signalling play a role in regulating the pool of beige adipose progenitors [20]. It has been shown that Smad2/3 pathway was active during hiPSC-BAP differentiation suggesting that bioactive TGFβ family members were secreted that might lock differentiation [14]. In agreement with this hypothesis, Su et al. [21] showed more recently that expression of TGFβ-ligands and receptors increased from the differentiation of FOXF1 mesoderm progenitors towards adipocytes during in vitro development of hiPSCs [21]. Then, the anti adipogenic role of the TGFβ pathway has been functionally demonstrated thanks to the use of the TGFβ inhibitor SB431542 [22]. Inhibition of active Smad 2/3 pathway upon SB431542 addition during hiPSC-BAP differentiation induced a dramatically increased of UCP1 expression and of the number of mature beige adipocytes [14,15,21,23]. In addition, inhibition of TGFβ signaling in hiPSC-mesenchymal stem cells, i.e., before induction of adipogenic differentiation, promoted the generation of adipocytes [21]. Altogether, these data underline the critical role of TGFβ pathway in the commitment of hiPSC into the adipogenic lineage. They indicate that TGFβ signalling inhibition enhances the conversion of mesenchymal stem cells into adipogenic progenitors and switches on the differentiation of progenitors into mature beige adipocytes.
INHIBITION OF TGFβ PATHWAY IS REQUIRED ONLY DURING THE FIRST DAYS OF DIFFERENTIATION OF hiPSC-3D BEIGE ADIPOSPHERES
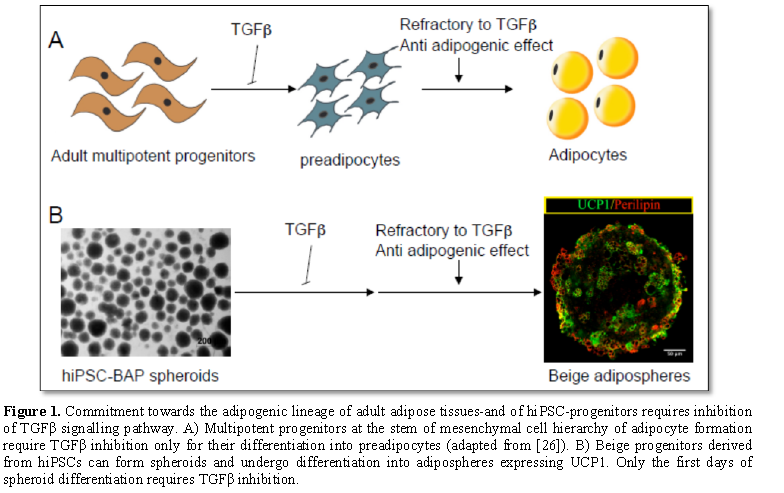
Inhibition of TGFβ pathway is required to induce differentiation of hiPSC-derived adipose progenitor cells into adipocytes, whereas is not for the differentiation of progenitors derived from human adult adipose tissues. The low hiPSC-BAP adipogenic capacity compared to adult-BAPs is reminiscent of an observation reported by Han et al. [24]. These authors observed that epididymal adipose tissue, which undergoes early development in mouse, is composed of progenitor cells that lack their adipogenic capacity once isolated from the tissue. In contrast to cells derived from other fat pads that developed later, epididymal fat cells required a 3D structure and a different micro-environment to undergo differentiation. Therefore, among the reasons to explain the weak efficacy of hiPSC-BAP differentiation, one can mention the culture conditions that do not mimic the phenotype of the cells and their physiological microenvironment within the adipose tissue. Cells are classically grown as monolayer, which poorly reflects the in vivo situation [25]. In contrast, the cell-cell and cell-extracellular matrix interactions are promoted in 3D configurations. Therefore, 3D cultures represent a bridge between traditional cell culture and live tissue. HiPSC-BAPs can form 3D spheroids able to differentiate into beige adipospheres expressing UCP-1 (Figure 1B, Yao X and Dani C, unpublished data). In fact, beige adipospheres revealed a TGFβ pathway depend phase only during the first days of spheroid differentiation.
CONCLUSION
Interestingly, Seale group has recently proposed a mesenchymal progenitor cell hierarchy in adipose tissue where the multipotent progenitor cell required inhibition of TGFβ pathway for its differentiation into preadipocytes. Then, preadipocytes are refractory to the anti adipogenic action of TGFβ to differentiate into adipocytes (Figure 1A, [26]). As discussed above, differentiation of hiPSC-BAP spheroids display also a sensitive and a refractory phase to anti adipgenic effect of TGFβ. It is tempting to speculate that BAPs derived from hiPSCs resemble the multipotent progenitor subpopulation in adult adipose tissue at the origin of preadipocytes. Further analyses are required to test this hypothesis. Numerous other issues have also to be solved before a therapeutic use of iPSCs in the obesity field, but identification of pathways governing the differentiation of BAPs at a high level as well as their capacity to form 3D adipospheres open the opportunity of using hiPSCs advantages for anti-obesity therapy.
ACKNOWLEDGEMENT
The work has been supported by the ANR-18-CE18-0006-01.
1. Cypess AM, White AP, Vernochet C (2013) Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med 19: 635-639.
2. Van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM (2009) Cold-activated brown adipose tissue in healthy men. N Engl J Med 360: 1500-1508.
3. Stanford KI, Middelbeek RJ, Townsend KL (2013) Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 123: 215-223.
4. Gunawardana SC, Piston DW (2010) Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes 61:674-682.
5. Liu X, Wang S, You Y (2015) Brown adipose tissue transplantation reverses obesity in Ob/Ob mice. Endocrinology 156: 2461-2469.
6. Min SY, Kady J, Nam M (2016) Human 'brite/beige' adipocytes develop from capillary networks and their implantation improves metabolic homeostasis in mice. Nat Med 22: 312-318.
1. Villarroya F, Gavalda-Navarro A, Peyrou M, Villarroya J, Giralt M (2017) The lives and times of brown adipokines. Trends Endocrinol Metab 28: 855-867.
2. Takahashi K, Tanabe K, Ohnuki M (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861-872.
3. Shi Y, Inoue H, Wu JC, Yamanaka S (2016) Induced pluripotent stem cell technology: A decade of progress. Nat Rev Drug Discov 16: 115-130.
4. Taura D, Noguchi M, Sone M (2009) Adipogenic differentiation of human induced pluripotent stem cells: Comparison with that of human embryonic stem cells. FEBS Lett 583: 1029-1033.
5. Ahfeldt T, Schinzel RT, Lee YK (2012) Programming human pluripotent stem cells into white and brown adipocytes. Nat Cell Biol 14: 209-219.
6. Mohsen-Kanson T, Hafner AL, Wdziekonski B (2014) Differentiation of human induced pluripotent stem cells into brown and white adipocytes: Role of Pax3. Stem Cells 32: 1459-1467.
7. Hafner Al, Dani C (2014) Human induced pluripotent stem cells: A new source for brown and white adipocytes. World J Stem Cells 6: 467-472.
8. Hafner Al, Contet J, Ravaud C (2016) Brown-like adipose progenitors derived from human induced pluripotent stem cells: Identification of critical pathways governing their adipogenic capacity. Sci Rep 6: 32490.
9. Anne-Laure Hafner TM-KACD (2016) A protocol for the differentiation of brown adipose progenitors derived from human induced pluripotent stem cells at a high efficiency with no gene transfer. Nature Protocol Exchange.
10. Phillips DJ (2005) Activins, inhibins and follistatins in the large domestic species. Domest Anim Endocrinol 28: 1-16.
11. Zamani N, Brown CW (2011) Emerging roles for the transforming growth factor-{beta} superfamily in regulating adiposity and energy expenditure. Endocr Rev 32: 387-403.
12. Zaragosi LE, Wdziekonski B, Villageois P (2010) Activin a plays a critical role in proliferation and differentiation of human adipose progenitors. Diabetes 59: 2513-2521.
13. Bourlier V, Sengenes C, Zakaroff-Girard A (2012) TGF-beta family members are key mediators in the induction of myofibroblast Phenotype of Human Adipose Tissue progenitor cells by macrophages. PLoS One 7: e31274.
14. Wankhade UD, Lee JH, Dagur PK (2018) TGF-beta receptor 1 regulates progenitors that promote browning of white fat. Mol Metab 16: 160-171.
15. Su S, Guntur AR, Nguyen DC (2018) A renewable source of human beige adipocytes for development of therapies to treat metabolic syndrome. Cell Rep 25: 3215-3228 e3219.
16. Inman GJ, Nicolas FJ, Callahan JF (2002) SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5 and ALK7. Mol Pharmacol 62: 65-74.
17. Hafner AL, Mohsen-Kanson T, Dani C (2018) Differentiation of brown adipocyte progenitors derived from human induced pluripotent stem cells. Methods Mol Biol 1773: 31-39.
18. Han J, Lee J, Jin J 2011) The spatiotemporal development of adipose tissue. Development 138: 5027-5037.
19. Horvath P, Aulner N, Bickle M (2016) Screening out irrelevant cell-based models of disease. Nat Rev Drug Discov 15: 751-769.
20. Merrick D, Sakers A, Irgebay Z (2019) Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science 364: 6438.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- Journal of Cardiology and Diagnostics Research (ISSN:2639-4634)
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- International Journal of AIDS (ISSN: 2644-3023)
- Journal of Renal Transplantation Science (ISSN:2640-0847)
- Journal of Immunology Research and Therapy (ISSN:2472-727X)